INDICATION & IMPORTANT SAFETY INFORMATION
INDICATION
IMPORTANT SAFETY INFORMATION
INDICATION
BLENREP is indicated in combination with bortezomib and dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least two prior lines of therapy, including a proteasome inhibitor and an immunomodulatory agent.
IMPORTANT SAFETY INFORMATION
WARNING: OCULAR TOXICITY
- BLENREP causes changes in the corneal epithelium resulting in changes in vision, including severe visual impairment, and symptoms such as blurred vision and dry eyes. In the clinical study, corneal ulcers, including cases with infection, also occurred.
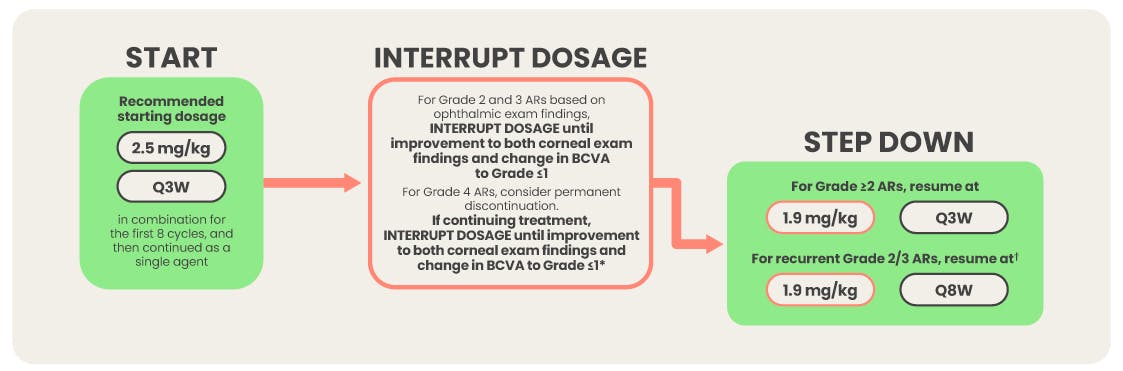
- Conduct ophthalmic exams at baseline, before each dose, promptly for new or worsening symptoms, and as clinically indicated. In the clinical study, 83% of patients required a dosage modification due to ocular toxicity. Withhold BLENREP until improvement and resume or permanently discontinue, based on severity.
- Because of the risk of ocular toxicity, BLENREP is available only through a restricted program called the BLENREP Risk Evaluation and Mitigation Strategy (REMS).
WARNINGS AND PRECAUTIONS
Ocular Toxicity
BLENREP causes ocular toxicity, defined as changes in the corneal epithelium and changes in BCVA based on ophthalmic exam (including slit lamp exam), or other ocular adverse reactions as defined by the CTCAE.
In DREAMM-7, ocular toxicity occurred in 92% of patients, including Grade 3 or 4 in 77% of patients. The most common ocular toxicities (>25%) were reduction in BCVA (89%) and corneal exam findings (86%) based on ophthalmic exam findings, blurred vision (66%), dry eye (51%), photophobia (47%), foreign body sensation in eyes (44%), eye irritation (43%), and eye pain (33%).
Ocular toxicity based on ophthalmic exam findings was reported as Grade 2 in 9% of patients, Grade 3 in 56% of patients, and Grade 4 in 21% of patients. The median time to onset of the first Grade 2 to 4 ophthalmic exam findings was 43 days (range: 15 to 611 days). The median duration of all Grade 2 to 4 ophthalmic exam findings was 85 days (range: 5 to 813 days). Patients experienced a median of 3 episodes (range: 1 to 11 episodes) of ocular toxicity based on ophthalmic exam findings. Of the patients with Grade 2 to 4 ophthalmic exam findings, 42% had improvement of the last event to Grade 1 or better; 22% had resolution of the last event based on return to baseline or normal ophthalmic exam findings.
The most commonly reported corneal exam findings included superficial punctate keratopathy, microcyst-like deposits, epithelial changes, and haze. Cases of corneal ulcer, including cases with infection, have been reported and should be managed promptly by an eye care professional.
A reduction in BCVA to 20/50 or worse in at least one eye occurred in 69% of patients, including 29% who experienced a change in BCVA to 20/100 or worse, and 12% who experienced a change in BCVA to 20/200 or worse. Of the patients with reduced BCVA to 20/50 or worse in at least one eye, 61% had resolution of the last event to baseline or better. Of the patients with reduced BCVA to 20/100 or worse, 57% had resolution of the last event. Of the patients with reduced BCVA to 20/200 or worse, 48% had resolution of the last event.
Ophthalmic exams (including slit lamp exam and BCVA assessment) should be conducted by an eye care professional, such as an ophthalmologist or optometrist, at baseline, before each dose of BLENREP, promptly for new or worsening symptoms, and as clinically indicated. Perform baseline exam within 4 weeks prior to the first dose. Perform each follow-up exam within 10 days prior to the next planned dose. All effort should be made to schedule the exam as close to BLENREP dosing as possible. Withhold BLENREP until improvement in both corneal exam findings and change in BCVA to Grade 1 or less and resume at same or reduced dose or permanently discontinue based on severity.
Counsel patients to promptly inform their healthcare provider of any ocular symptoms. Counsel patients to use preservative-free artificial tears at least 4 times a day starting with the first infusion and continuing until the end of treatment, and to avoid wearing contact lenses for the duration of therapy. Bandage contact lenses may be used under the direction of an eye care professional.
Changes in visual acuity may be associated with difficulty for driving and reading. Counsel patients to use caution when driving or operating machinery.
BLENREP Risk Evaluation and Mitigation Strategy (REMS)
BLENREP is available only through a restricted program called the BLENREP REMS because of the risk of ocular toxicity.
Further information is available at www.BLENREPREMS.com and 1-855-690-9572.
Thrombocytopenia
Thrombocytopenia of any grade occurred in 100% of patients in DREAMM-7.
Grade 2 thrombocytopenia occurred in 10% of patients, Grade 3 in 29% of patients, and Grade 4 in 45% of patients. Clinically significant bleeding (Grade ≥2) occurred in 7% of patients with concomitant low platelet levels (Grade 3 or 4).
Monitor complete blood cell counts at baseline and periodically during treatment as clinically indicated. Withhold or reduce the dose of BLENREP based on severity.
Embryo-fetal Toxicity
Based on its mechanism of action, BLENREP can cause fetal harm when administered to a pregnant woman because it contains a genotoxic compound (the microtubule inhibitor, monomethyl auristatin F [MMAF]) and it targets actively dividing cells.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with BLENREP and for 4 months after the last dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with BLENREP and for 6 months after the last dose.
ADVERSE REACTIONS
The most common adverse reactions (≥20%) with BLENREP in combination with bortezomib and dexamethasone are reduction in BCVA, corneal exam findings, blurred vision, dry eye, photophobia, foreign body sensation in eyes, eye irritation, upper respiratory tract infection, hepatotoxicity, eye pain, diarrhea, fatigue, pneumonia, cataract and COVID-19.
The most common Grade 3 or 4 (≥10%) laboratory abnormalities are decreased platelets, decreased lymphocytes, decreased neutrophils, increased gamma-glutamyl transferase, decreased white blood cells, and decreased hemoglobin.
Please see full Prescribing Information, including Boxed Warning.